Last update and review: July 18, 2020.
Short summary.
In several cases where chronic inflammation was suspected, we have been seeing positive results with a “pro-resolution” approach. “Pro-resolution” differs form “anti-inflammation”. In the article below, we share some of our notes and comments on the subject.
“The state of the art in the biology of resolution of inflammation” according to Fullerton and Gilroy, 2016 (1).
Here is how Fullerton and Gilroy describe the difference in their recent review (Fullerton and Gilroy, 2016 (1)):
It is important to emphasize the difference between anti-inflammation (Figure 3, part a) and pro-resolution (Figure 3, part b). The former describes the inhibition of factors that drive inflammation, including vasoactive amines, eicosanoids, cytokines, chemokines and cell adhesion molecules. Among the pharmacological tools developed to achieve this clinically are non-steroidal anti-inflammatory drugs and biologicals such as anti-tumour necrosis factor (TNF) therapies. This form of intervention dampens inflammation from the onset, with or without accelerated resolution. By comparison, pro-resolution describes enhancing or promoting the factors essential for removal of the inciting stimulus as well as dampening pro-inflammatory signalling, followed by leukocyte clearance. It is envisioned that pro-resolution therapies will not necessarily affect onset but will accelerate resolution.
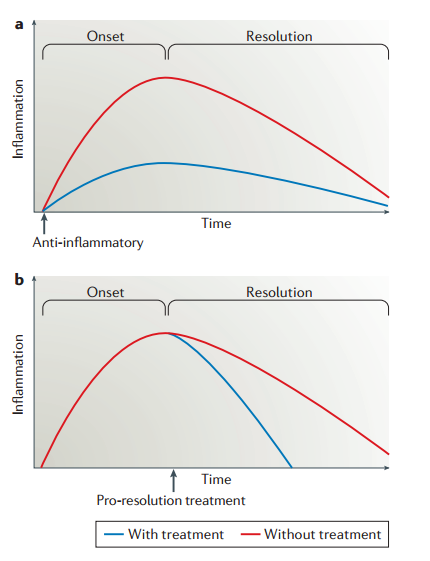
COVID-19 contradicts the picture of non-resolving inflammation presented by Fullerton and Gilroy.
There is another figure on the resolution phase of inflammation in the paper by Fullerton and Gilroy, 2016 (1). We are, however, skeptical about the “part b” in the “Figure 1” which shows non-resolving inflammation.

Fullerton and Gilroy (1) write:
…diseases driven by ‘inflammation gone wrong’ may arise from incomplete resolution of the initial acute response that, in turn, does not fully engage an appropriate adaptive immune response that would otherwise lead to full resolution.
The statement above seems to contradict the picture that we observe in COVID-19. Indeed, we learned that in COVID-19, patients who develop severe disease produce higher levels of anti-bodies than patients with milder forms of COVID-19. Severe COVID-19 is associated with inflammatory and immune dysfunction which apparently occurs despite a strong response of the adaptive immune system.
High IgG antibody levels in patients with severe “hyper-inflammatory” forms of COVID-19 .
A pre-print from a group from Switzerland (2) shows that, in severe COVID-19, by day 20 since symptom onset, everybody, including a large proportion of prone to inflammatory and immune dysfunction 80- and even 90+ year-olds, develop IgG antibodies that bind to the spike protein of SARS-CoV-2. Note the high IgG antibody levels in patients with severe forms of COVID-19.
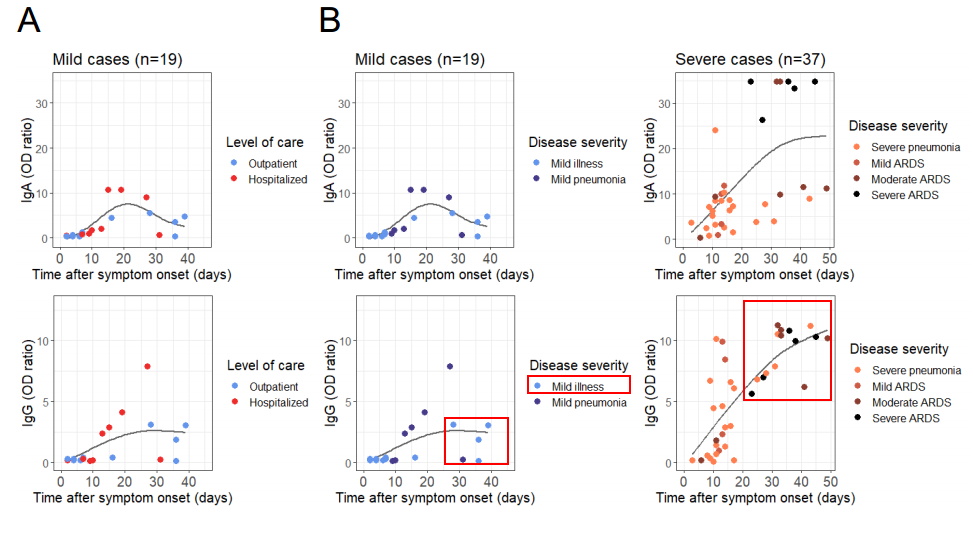
COVID-19: “IgG, immunoglobulin M (IgM) and IgA titres were significantly higher in the poor clinical outcome group“
Another evidence that inflammatory dysfunction in COVID-19 develops despite the presence of the adaptive immune response is provided by the study by Lagier et al., 2020 (3). Indeed, detectable IgG antibodies at baseline were associated with poor outcomes.

Lagier et al., 2020 (2):
Serology was performed in 2,302 patients. Immunoglobulin G (IgG) to SARS-CoV-2 was detected in 726 patients (31.5%). Immunoglobulin A (IgA) was detected in 12.9% of patients under 65 years with poor clinical outcome, compared to 2.3% of patients with good clinical outcome (p<0.05) (Table S2). IgG, immunoglobulin M (IgM) and IgA titres were significantly higher in the poor clinical outcome group (Table S3). Surprisingly, we observed an increase in seroprevalence and specific antibody titres in patients with poor clinical outcome during evolution (data not shown) [20].
Selected references:
1. Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15(8):551-567. doi:10.1038/nrd.2016.39
2. Cervia et al. Systemic and mucosal antibody secretion specific to SARS-CoV-2 during mild versus severe COVID-19. Pre-print.
https://www.biorxiv.org/content/10.1101/2020.05.21.108308v1
Accessed on July 18, 2020.
3. Lagier et al. Travel Medicine and Infectious Disease(2020).