Last update and review: August 21, 2021.
A short summary.
Sheikh-Mohamed, 2021 (1), tested saliva of mRNA- and adenovirus/mRNA-vaccinated subjects. They detected IgG and IgA anti-Spike and anti-RBD (receptor binding domain of the Spike-protein). Titers of saliva IgG were “high” in vaccinated in comparison to COVID-19 patients. However, neutralizing activity of the saliva samples from vaccinated subjects was NOT superior to that of the pre-vaccination pre-pandemic saliva. Secretory IgA may have been produced because Spike-protein fabricated by the infected cells of the vaccinated reached salivary glands. In some subjects, there were a lot of IgA. A lot of Spike-protein of vaccine origin must have reached their salivary glands. And other organs. Notably, testes, ovaries, where vaccine particles were detected in animal and human studies.
We learn nothing encouraging about the current COVID-19 mRNA and vector vaccines. However, we may be learning that human saliva is good at neutralizing viruses WITHOUT any vaccination and virus-specific antibodies.

A study by Sheikh-Mohamed et al., 2021.
Subjects and saliva samples.
Sheikh-Mohamed, 2021 (1): “Saliva samples from 150 long-term care home (LTCH) workers who received either BNT162b2 or mRNA-1273.”
A dubious cut-off.
Sheikh-Mohamed, 2021 (1): “Saliva from 51 participants 75 that were collected pre-pandemic or during the early months of the pandemic in a low prevalence 76 region were pooled, and a positive was established that was 2 standard deviations above 77 the median for these negative samples.”
94% and 41% of participants (vaccinated but without signs of previous infection) with were positive for anti-Spike IgG 82 and IgA
Sheikh-Mohamed, 2021 (1): “When we analyse only those participants who had no evidence of prior SARS-CoV-2 81 infection (n=107), we found that 94% and 41% of participants were positive for anti-Spike IgG 82 and IgA, and 93% and 20% of participants, were positive for anti-RBD IgG and IgA antibodies in 83 their saliva (Fig 1). Furthermore, anti-Spike and anti-RBD IgG and IgA levels correlated well with 84 the level of these antibodies in the blood (Supplementary Fig. 1), suggesting that antibodies 85 detected in the saliva after 2 doses of mRNA COVID-19 vaccine were at least in part derived from 86 the systemic immune response.”
Male sex had a negative independent association with the salivary anti-Spike IgG 89 response (Supplementary Table 3b) as has been observed before for other vaccines.
Sheikh-Mohamed, 2021 (1): “In multivariable analysis, age and prior SARS-CoV-2 infection 87 were independently associated with the salivary anti-Spike IgA response (Supplementary Table 88 3a). In contrast, male sex had a negative independent association with the salivary anti-Spike IgG 89 response (Supplementary Table 3b) as has been observed before for other vaccines 12.”
Higher salivary IgA after a single dose of mRNA (Pfizer, BNT1162b2).
“These subjects received 1 dose of BNT1162b2.” “Focusing on IgG and IgA (in subjects after a single dose) we observed that 97% and 93% of participants were positive 107 for anti-Spike IgG and IgA.”
Unexpected: Salivary IgA dropped after the 2nd dose.
Sheikh-Mohamed, 2021 (1): “Curiously, in contrast to IgG, anti-Spike and anti-RBD IgA levels were higher 2 weeks after dose 1 compared to 2 weeks after dose 2.”
Neutralizing activity of salive at two-fold dilutions was tested against a pseudovirus with SARS-CoV-2 Spike: no difference with pre-pandemic saliva.
Sheikh-Mohamed, 2021 (1): “To assess if either COVID- 164 19 vaccine provokes neutralizing antibodies (nAb) (Sloppy phrasing. Should be “results in neutralizing activity) against SARS-CoV-2 in the oral cavity, we tested saliva from the MSB-1 and MSB-2 cohorts in a pseudovirus entry assay. Specifically, saliva at two-fold dilutions was added to hACE2-mCherry expressing HEK293 cells that were co- incubated with recombinant Vesicular Stomatitis Virus (rVSV)-eGFP-SARS-CoV-2-Spike. However, when FR NT50 or FR NT70 values was tabulated across the cohort, statistical significance was not achieved compared to (pre-pandemic) baseline.”
Spike-protein generated by vaccination may reach salivary glands and many other organs.
After vaccination, infected cells produce Spike-protein that can be detected in plasma with ultra-sensitive technique. One explanation of why anti-Spike and anti-RBD (receptor binding domain) sIgA are generated in the saliva after intramuscular vaccination is that plasma-associated Spike antigen may reach the salivary glands. The corollary of the above is that Spike-protein of the same origin may reach other organs. This increases the risk of autoimmune or chronic inflammatory complication post-vaccination. This said, viral mRNA of SARS-CoV-2 has been found in many organs of infected laboratory animals. In people with past COVID-19 infection, viral mRNA and macrophages infected with Spike-protein have been found months after the initial infection.  Sheikh-Mohamed, 2021 (1): “It is unclear how sIgA anti-Spike/RBD are generated in the saliva following intramuscular (IM) immunization with BNT162b2 or mRNA-1273. Of note, Spike protein can be detected in the plasma, increasing one to 5 days after mRNA-1273 vaccination using an ultra-sensitive detection technique. Thus one possibility is that plasma-associated Spike antigen may reach the salivary glands (which are surrounded by capillaries), provoking a local sIgA response. We further hypothesize that the decline in anti-RBD sIgA at dose 2 may be due to rapid opsonization of Spike antigen by the exceedingly high levels of pre-existing serum anti-Spike IgG induced by dose 1. While beyond the scope of this study, using a highly sensitive assay such as Simoa® to measure Spike antigen in the saliva 1-5 days post-dose 1 versus post-dose 2 (which we did not collect) would be a logical next step to test this hypothesis. Another possibility is that a mucosal IgA response to mRNA 205 vaccination takes place in the gut, and plasma cells generated at this location leave the gut (as we 206 have shown before 18) and disseminate to other mucosal surfaces, including the salivary glands.”
Sheikh-Mohamed, 2021 (1): “It is unclear how sIgA anti-Spike/RBD are generated in the saliva following intramuscular (IM) immunization with BNT162b2 or mRNA-1273. Of note, Spike protein can be detected in the plasma, increasing one to 5 days after mRNA-1273 vaccination using an ultra-sensitive detection technique. Thus one possibility is that plasma-associated Spike antigen may reach the salivary glands (which are surrounded by capillaries), provoking a local sIgA response. We further hypothesize that the decline in anti-RBD sIgA at dose 2 may be due to rapid opsonization of Spike antigen by the exceedingly high levels of pre-existing serum anti-Spike IgG induced by dose 1. While beyond the scope of this study, using a highly sensitive assay such as Simoa® to measure Spike antigen in the saliva 1-5 days post-dose 1 versus post-dose 2 (which we did not collect) would be a logical next step to test this hypothesis. Another possibility is that a mucosal IgA response to mRNA 205 vaccination takes place in the gut, and plasma cells generated at this location leave the gut (as we 206 have shown before 18) and disseminate to other mucosal surfaces, including the salivary glands.”
50% diluted saliva of vaccinated subjects, with IgG and some IgA in it, had no neutralizing activity in comparison to pre-epidemic saliva.
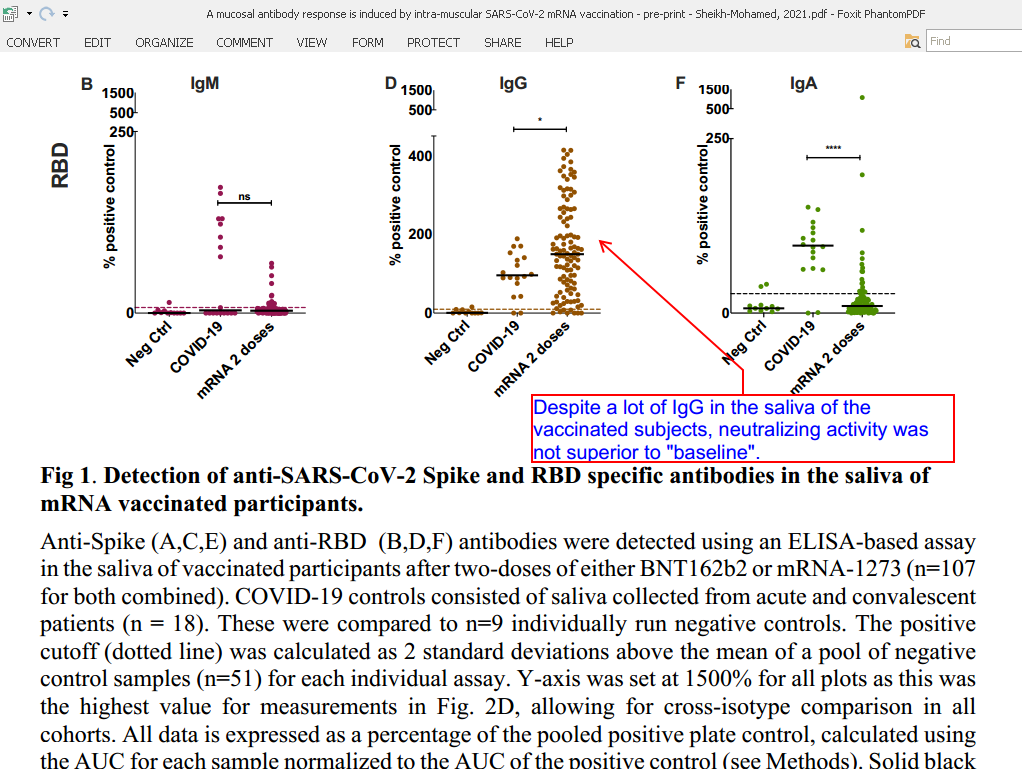 Sheikh-Mohamed, 2021 (1): “Lastly, while both MSB-1 participants’ and MSB-2 participants’ saliva showed hints of neutralizing activity, when averaged across the cohort as an FR NT50 or FR NT70 value, this did not reach statistical significance over baseline samples. The reason for this may be technical – anti-viral 226 properties of saliva beyond antibodies (i.e. enzymes) may impact the ability of rVSV-eGFP- 227 11 SARS-CoV-2-Spike to infect hACE2 expressing HEK293 cells, thus introducing background into the assay.”
Sheikh-Mohamed, 2021 (1): “Lastly, while both MSB-1 participants’ and MSB-2 participants’ saliva showed hints of neutralizing activity, when averaged across the cohort as an FR NT50 or FR NT70 value, this did not reach statistical significance over baseline samples. The reason for this may be technical – anti-viral 226 properties of saliva beyond antibodies (i.e. enzymes) may impact the ability of rVSV-eGFP- 227 11 SARS-CoV-2-Spike to infect hACE2 expressing HEK293 cells, thus introducing background into the assay.”
Conclusions.
neutralizing activity of the saliva samples from vaccinated subjects was NOT superior to that of the pre-vaccination pre-pandemic saliva. Secretory IgA may have been produced because Spike-protein fabricated by the infected cells of the vaccinated reached salivary glands. In some subjects, there were a lot of IgA. A lot of Spike-protein of vaccine origin must have reached his or her salivary glands. And other organs. Notably, testes, ovaries, were at least vaccine particles were detected in animal and human studies. We learn nothing encouraging about the current COVID-19 mRNA and vector vaccines. However, we may be learning that human saliva is good at neutralizing viruses WITHOUT any vaccination and virus-specific antibodies.
Selected references:
1. Sheikh-Mohamed et al., 2021. A mucosal antibody response is induced by intra-muscular SARS-CoV-2 mRNA vaccination. Pre-print. Available at: https://doi.org/10.1101/2021.08.01.21261297 Accessed on August 21, 2021.



