The last review and update: August 24, 2021.
A short summary.
Our current view, as of August 2021, is that only for the most vulnerable individuals, the benefits of the current vaccines may outweigh the associated risks. The vulnerable are defined as those who can’t, for different reasons, take any other protection measure. We do NOT currently recommend ANY of the available COVID-19 vaccines to the generally competent normal people. The risks related to the currently available vaccines are very high. Chimerical adenovirus vector vaccines, mRNA vaccines, and deactivated virus vaccines against SARS-CoV-2 all contain elements that can provoke long-term inflammatory and autoimmune reactions, severe adverse effects, e.g. coagulopathy. Spike-protein of SARS-CoV-2 is not an inert particle. It is highly inflammatory and toxic. Viral mRNA can be integrated into human genome. And though several vaccines were effective in phase-3 trials by protecting against hospitalization, this protection turns out to be short-lived, limited to 2 to 7 months after vaccination. SARS-CoV-2 infection can be avoided rather easily, and it can be treated effectively by literate members of public. Be literate at all times.
A review of Johnson & Johnson’s COVID-19 adenovirus vector vaccine.
A review of Johnson & Johnson’s COVID-19 adenovirus vector vaccine based on the press-release of the phase 3 trial results from January 29, 2021, and the study design protocol.
The review is in the form of thread originally published on a social network.
The verdict (updated on August 24, 2021):
The verdict (updated on August 24, 2021): Johnson & Johnson’s COVID-19 adenovirus vector vaccine was rather effective in phase-3 trials at preventing severe COVID-19. However, the real-life data show that the protection is short-lived, limited to 2 to 7 months after vaccination. The single-dose Johnson and Johnson vector vaccine creates the shortest period of protection. None of the intramuscular vaccines against COVID-19 limit transmission. For the most vulnerable groups, in particular, people of advanced age who can not take other prevention, hygiene, prophylaxis, and early treatment measures, Astra-Zeneca-Oxford vector vaccine, two doses, may be a somewhat better option. Tens of thousands of people of advanced age vaccinated in Scotland apparently survived vaccination with Astra-Zeneca-Oxford. Adverse effects of Astra-Zeneca-Oxford vaccine are less pronounced than in younger groups. For younger people, and competent literate normal people of all ages, we do NOT recommend Astra-Zeneca-Oxford vaccine (as of August 2021).
The thread on Johnson & Johnson’s COVID-19 adenovirus vector vaccine.
1/n
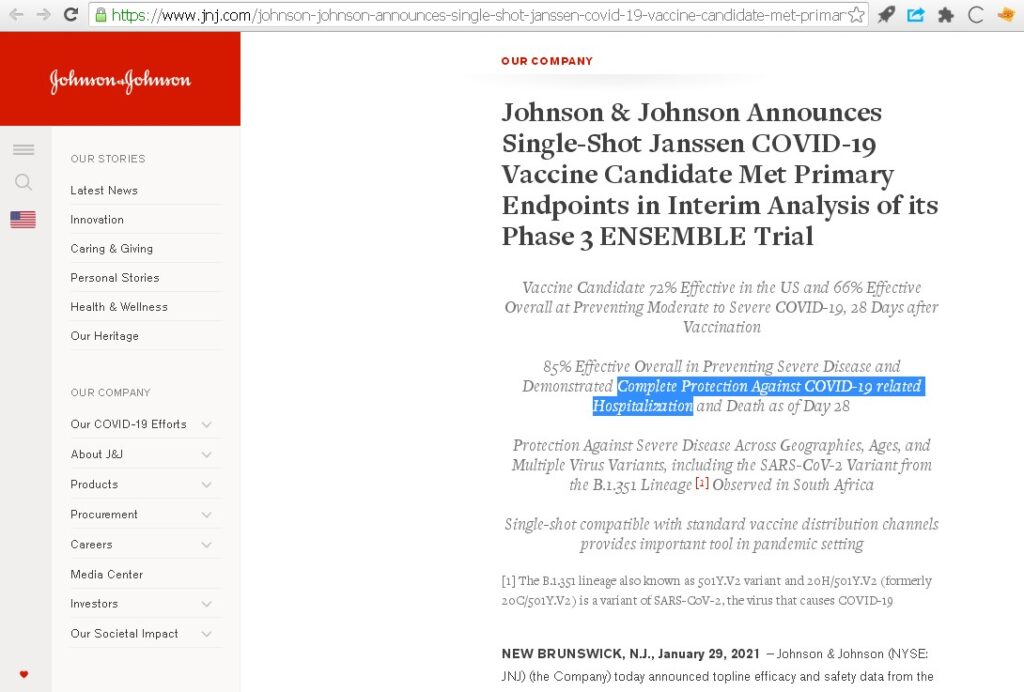
Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine. J&J employs competent writers for their press-releases and reports on important data:
“Complete Protection Against COVID-19 related Hospitalization and Death as of Day 28.”
2/n Well, the writers employed by johnson and Johnson are not as competent as we hoped: “abnormal blood oxygen saturation above 93%”. Analysis: Healthy people have blood oxygen saturation ABOVE 93%.
“Big Pharma” can’t even write correct press-releases.

3/n “Respiratory rate” is normal up to 22 per minute. Johnson and Johnson does not know it or its writers make errors.

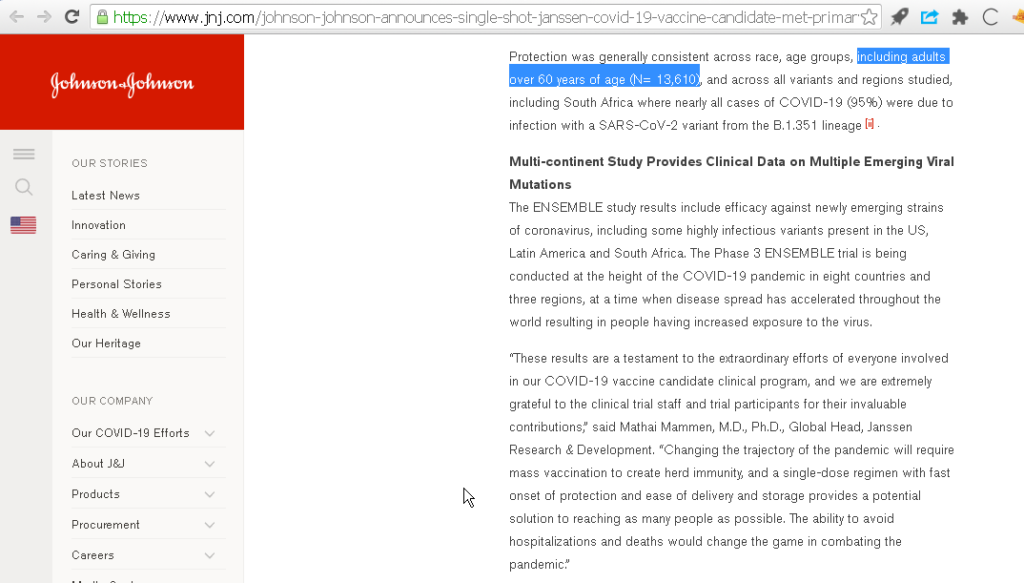
4/n Johnson and Johnson had 13k adults aged above 60 in its trials. This is good.
“including adults over 60 years of age (N= 13,610)”
5/n “The safety profile was consistent with other vaccine candidates using Janssen’s AdVac® technology among more than 200,000 people to date. Overall fever rates were 9% and Grade 3 fever 0.2%”

6/n Typical idiotic writing by “Big Pharma”:
“Janssen’s single-dose vaccine candidate is estimated to remain stable for two years at -20°C (-4°F), at least three months OF WHICH (???) can be at temperatures of 2-8°C (36°F–46°F).”

7/n Johnson and Johnson: “The trial, conducted in eight countries across three continents, includes a diverse and broad population including 34% (N= 14,672) of participants over age 60.”
Analysis: A design superior to that of Astra Zeneca – Oxford. @ajpollard1

8/n Johnson and Johnson: “41% of participants had comorbidities: (overall 41%), obesity (28.5%), type 2 diabetes (7.3%), hypertension (10.3%), HIV (2.8%);”
Analysis: It would be interesting to see if in unvaccinated there is, indeed, increased risk.
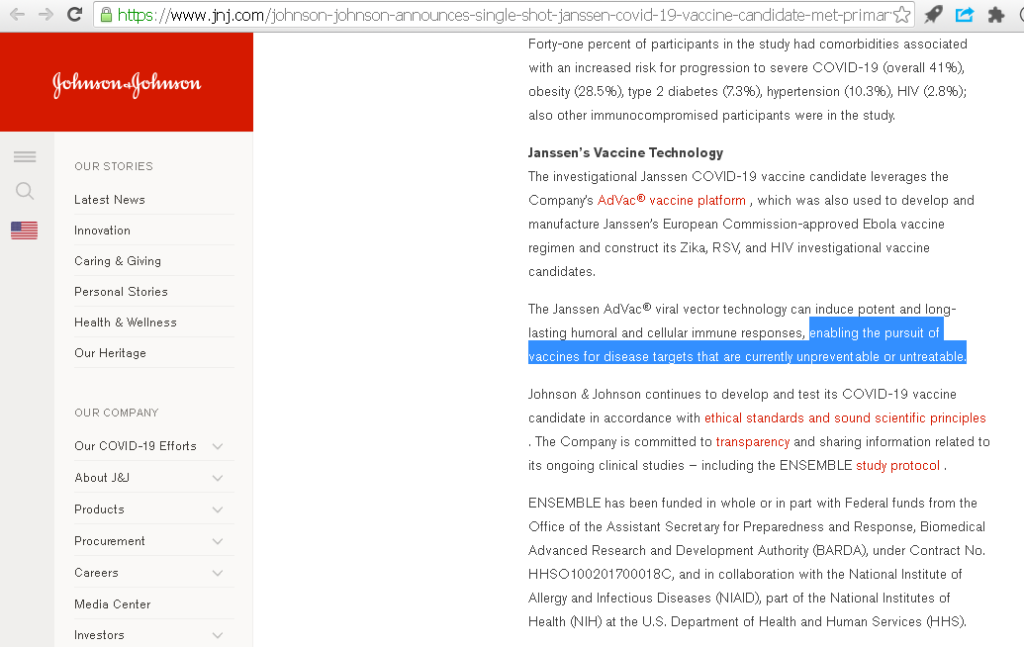
9/n Johnson and Johnson: “enabling the pursuit of vaccines for disease targets that are currently unpreventable or untreatable.”
Analysis: COVID-19 is both PREVENTABLE and treatable. No vaccine is needed for people with normal intelligence.
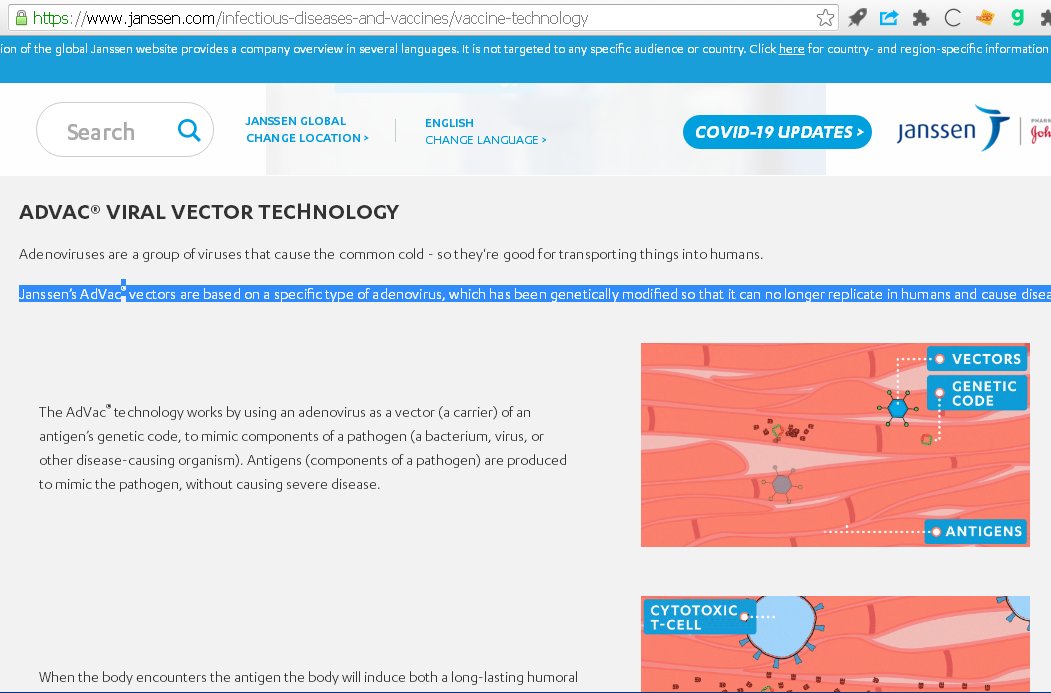
10/n “Janssen’s AdVac® vectors are based on a specific type of adenovirus, which has been genetically modified so that it can no longer replicate in humans and cause disease.”
Analysis: Viruses can recombine with other viruses. So, it is not totally safe, just as with AZ-Oxford.
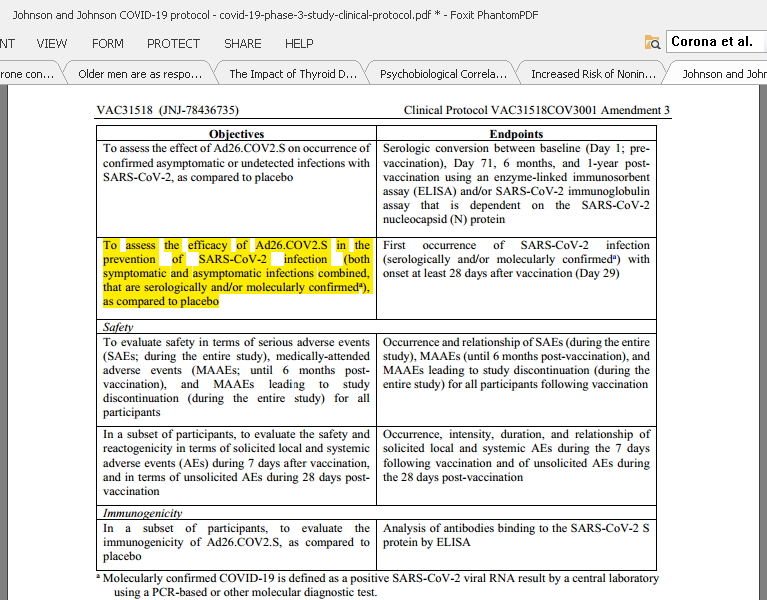
11/n Johnson and Johnson: “To assess the efficacy of Ad26.COV2.S in the prevention of SARS-CoV-2 infection (both symptomatic and asymptomatic infections combined, that are serologically and/or molecularly confirmed), as compared to placebo.”
But will they report it?
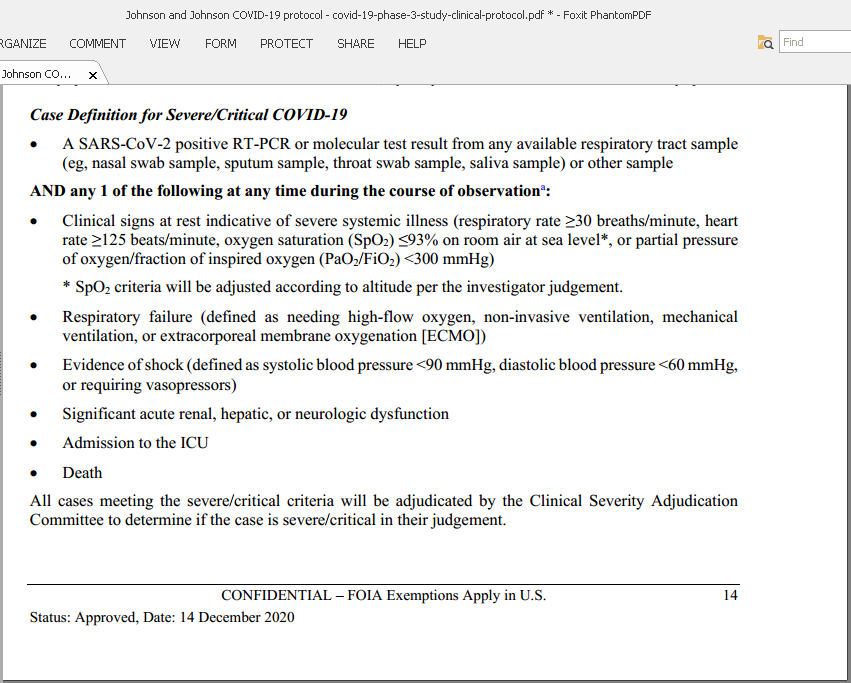
12/n The case definition for “moderate COVID-19” is almost completely bogus: E.g. normal respiratory rate is up to 22 breaths/min, but J and J will count 20 as disease.
Also headache+muscle pain and a PCR+ for J&J = “moderate COVID-19” which is wrong. It is almost “asymptomatic”.
13/n The case definitions based on a positive RT-PCR test is problematic since the sensitivity of RT-PCR tests is below 70% even during the most symptomatic phase of COVID-19.
Johnson and Johnson is affected. But “no hospitalizations” is a good result if true.
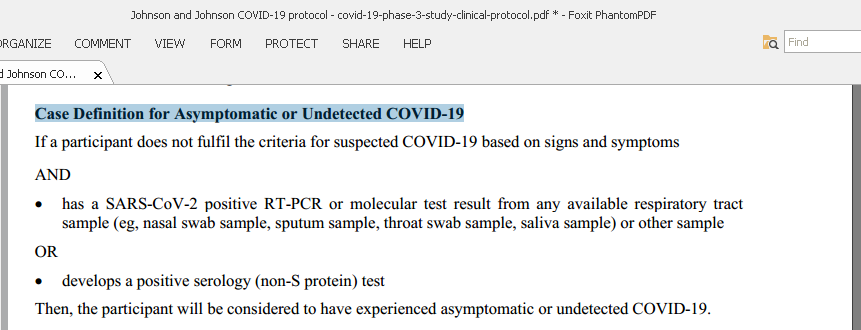
14/n Case Definition for Asymptomatic or Undetected COVID-19 in Johnson and Johnson study protocol: positive serology for non-S protein or a positive RT-PCR.
15/n Johnson and Johnson rely heavily on a positive RT-PRC, asks subjects to do “COVID-19 procedures”, which are mainly self assessing with a questionaire.
Analysis: In our counsultancy, we observe that people often struggle with questionnaires.
16/n Johnson and Johnson did not plan regular swabs to detect asymptomatic cases. This is a major flaw of the study design. The trial is financed by the US money printing press, and bureaucrats should have demanded weekly swabs in a subgroup of subjects.
17/n Johnson and Johnson: “Ad26.COV2.S (previously Ad26COVS1) is a monovalent vaccine composed of a recombinant, replication-incompetent adenovirus type 26 (Ad26) vector, constructed to encode the severe acute respiratorysyndrome coronavirus-2 (SARS-CoV-2) spike (S) protein.”

Originally posted by The New Neander’s Medical (@NNeanderMedical) on January 29, 2021.